Social stressors associated with age-related T lymphocyte percentages in older US adults: Evidence from the US Health and Retirement Study
Published: 06/13/2022, Proceedings of the National Academy of Sciences of the United States of America Vol. 119 | No. 25
Background
This study focuses on social stressors, that is, difficult or challenging circumstances1 that arise from social position and experience that are expected to be stressful, that occur in adulthood. Past research has categorized stressors in various ways, often depending on discipline; however, stressors are typically distinguished by timescale (e.g., acute, daily hassles, life events, chronic stressors) 2. It distinguishes discrimination-related stressors from other stressors because past research suggests these stressors have distinct, independent effects on health 3, 4, 5. It also distinguishes traumatic life events from other stressors, as is common in past research 2, 6, 7. Thus, it assesses five stress variables that have been well established past research and are available in a large number of studies of health and aging (viz., stressful life events, chronic stress, everyday discrimination, lifetime discrimination, and life trauma).
Social stress can modify the immune system in several ways, including increased inflammatory signaling and reduced antiviral responses 1, 8-10, suggesting that social stress may accelerate immune aging 11-21. However, past research has utilized small clinical and specialized samples, potentially limiting power to detect effects, power to assess mediators, and generalizability of results. In this study, a nationwide sample of 5,641 US adults over the age of 50 is utilized to assess associations between five categories of social stress that have well established effects on heath and enumerative measures of immunological aging, including naïve CD4+ T cell percentages (CD4+/CD3+/CD19−/CD45RA+/CCR7+/CD28+) naïve CD8+ T cell percentages (CD8+/CD3+/CD19−/CD45RA+/CCR7+/CD28+), terminally differentiated effector memory CD4+ T cell percentages (TemRA; CD4+/CD3+/CD19−/CD45RA+/CCR7−/CD28−), terminally differentiated effector memory CD8+ T cell percentages (CD8+/CD3+/CD19−/CD45RA+/CCR7−/CD28−), and the ratio of CD4+ to CD8+ T cells. Analyses also assessed potential mediation of these associations by socioeconomic and lifestyle factors and cytomegalovirus (CMV) seropositivity.
Study Design
This study utilizes data from the US Health and Retirement Study (HRS) 2016 Venous Blood Study (n = 9,934). Multiparameter flow cytometry was used to assess counts and percentages of 24 different types of immune cells using the standardized protocol by the Human Immunology Project 22 with minor modifications performed on an LSRII or a Fortessa ×20 flow cytometer (BD Biosciences).
It utilizes five well-established health-relevant measures of social stress: stressful life events 23, chronic stress 24, everyday discrimination 24, lifetime discrimination 25, and life trauma 26. Because everyday discrimination, stressful life events, lifetime discrimination, and life trauma assess life course or ongoing exposure to stress, it uses the first available data from the years these measures were assessed (2006 to 2016 for everyday discrimination, 2008 to 2012 for stressful life events, and 2006 to 2012 for lifetime discrimination and life trauma). Because this study has interest in assessing ongoing chronic stress, it only uses data from the 2014 and 2016 leave behind questionnaire.
Socioeconomic and lifestyle factors were assessed using self-reports of education (categorized: 0 to 11 y, 12 y, 13 to 15 y, and 16+ years as the reference), self-reported smoking (categorized: current smoker, past smoker, and nonsmoker as the reference), BMI (categorized: ≥ 25 and < 30 overweight, ≥ 30 and < 35 obese 1, ≥ 35 obese 2, and < 25 normal and underweight as the reference), and alcohol use (categorized: 1 to 4 drinks per day drinking, 5+ drinks per day drinking, and nondrinker as the reference).
CMV seropositivity was assessed using IgG antibodies in serum with the Roche e411 immunoassay analyzer (Roche Diagnostics Corporation) (categorized: borderline, reactive, or nonreactive as the reference) 27.
In all models the study controls for chronological age, race/ethnicity (non-Hispanic Black, Hispanic, bon-Hispanic other race, and non-Hispanic White as reference), and sex (male as reference).
Results
The weighted sample is 55.2% female and has a median age of 68 y. Of the sample, 84.9% is non-Hispanic White, 6.9% is non-Hispanic Black, 5.8% is Hispanic, and 2.5% is non-Hispanic other race; 10.1% has less than 12 y of education, 30.7% has 12 y of education, 26.1% has 13 to 15 y of education, and 33.1% has 16 or more years of education. Of the sample, 9.4% are current smokers and 45.4% are former smokers, more than one-third of the sample is obese (36.1%), and 53.6% were nondrinkers. On average, participants reported relatively low everyday discrimination, 0.50 stressful life events, 0.65 incidents of lifetime discrimination, 1.09 life traumas, and a moderate level of chronic stress. There was substantial variance on all of these scales.
Results from nested analyses regressing T cell subset percentage/ratio on each stressor while controlling for age, sex, race/ethnicity, and various potential mediators, are shown below.
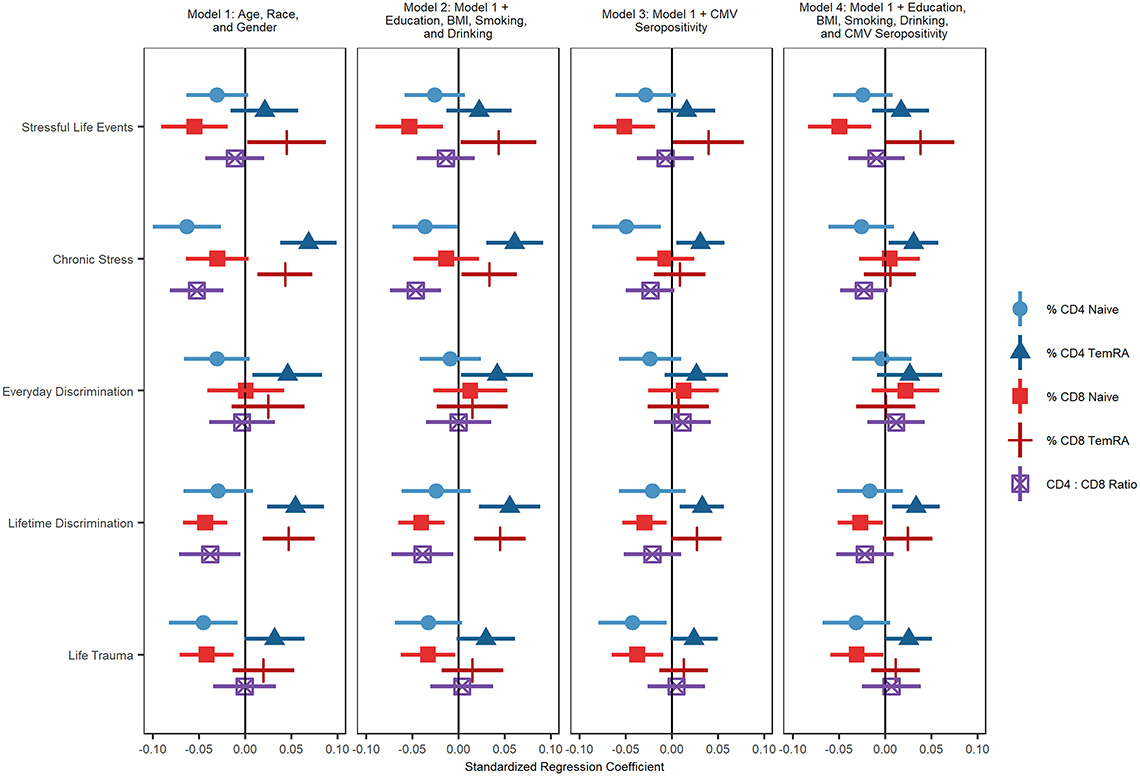
The association between terminally differentiated CD8+ T cells and more stressful life events was reduced to nonsignificance after controlling for education, BMI, smoking, drinking and CMV seropositivity. The association between stressful life events and CD8+ naïve T cell percentage remained statistically significant after controlling for all covariates. Experiencing greater chronic stress was related to a greater percentage of terminally differentiated CD4+ and terminally differentiated CD8+ T cells, a lower percentage of CD4+ naïve T cells, and a lower ratio of CD4+ to CD8+ T cells.
The association between chronic stress and CD4+ naïve T cells became nonsignificant after controlling for socioeconomic and lifestyle factors and CMV seropositivity. The association between chronic stress and terminally differentiated CD4+ T cells remained significant even after adjusting for education, BMI, smoking, drinking, and CMV seropositivity. The association between terminally differentiated CD8+ T cells and greater lifetime discrimination was reduced to nonsignificance after controlling for education, BMI, smoking, drinking, and CMV seropositivity. The associations between lifetime discrimination and terminally differentiated CD4+ and CD8+ naïve T cell percentages were both substantially reduced by these mediators but remained statistically significant.
Experiencing more everyday discrimination was related to having a higher percentage of terminally differentiated effector memory CD4+ T cells. This association was reduced after accounting for CMV seropositivity. Finally, experiencing a greater number of life traumas was related to a smaller percentage of CD4+ naïve T cells and CD8+ naïve T cells. The association between CD4+ naïve T cells and trauma was reduced to nonsignificance after controlling for education, BMI, smoking, and drinking, suggesting that these socioeconomic and lifestyle factors may mediate this association.
Associations between life trauma and CD8+ naïve T cells remained significant after including all covariates. There was only one statistically significant interaction. The effect of experiencing more lifetime discrimination on naïve CD8+ T cell percentage was amplified for Hispanic respondents compared to non-Hispanic White respondents.
Conclusions
Despite its limitations, this study provides important insights on the role of social stress in immune aging, highlighting a key role for health behaviors and social-environmental conditions as correlates of naïve T cell decline as well as a distinctive association of stressors with higher terminally differentiated CD4+ T cell percentages (i.e., independent of variables controlled for here). These results raise the possibility that interventions such as CMV vaccination and senolytic therapies might potentially help reduce social disparities in T cell immunologic aging 28. Interventions aimed at reducing stress or increasing resilience may be needed to address these inequalities.
References
- R. G. Reed, Stress and immunological aging. Curr. Opin. Behav. Sci. 28, 38–43 (2019).
- E. S. Epel et al., More than a feeling: A unified view of stress measurement for population science. Front. Neuroendocrinol. 49, 146–169 (2018).
- A. T. Geronimus et al., Do US Black women experience stress-related accelerated biological aging? A novel theory and first population-based test of Black-White differences in telomere length. Hum. Nat. 21, 19–38 (2010).
- A. T. Geronimus, M. Hicken, D. Keene, J. Bound, “Weathering” and age patterns of allostatic load scores among blacks and whites in the United States. Am. J. Public Health 96, 826–833 (2006).
- R. L. Simons et al., Discrimination, segregation, and chronic inflammation: Testing the weathering explanation for the poor health of Black Americans. Dev. Psychol. 54, 1993–2006 (2018).
- M. Levine, Traumatic life experiences are associated with increases in epigenetic aging. Biol. Psychiatry 83, S92–S93 (2018).
- M. E. Levine, S. W. Cole, D. R. Weir, E. M. Crimmins, Childhood and later life stressors and increased inflammatory gene expression at older ages. Soc. Sci. Med. 130, 16–22 (2015).
- R. Glaser, J. K. Kiecolt-Glaser, Stress-induced immune dysfunction: Implications for health. Nat. Rev. Immunol. 5, 243–251 (2005).
- J.-P. Gouin, L. Hantsoo, J. K. Kiecolt-Glaser, Immune dysregulation and chronic stress among older adults: A review. Neuroimmunomodulation 15, 251–259 (2008).
- J.-P. Gouin, R. Glaser, W. B. Malarkey, D. Beversdorf, J. Kiecolt-Glaser, Chronic stress, daily stressors, and circulating inflammatory markers. Health Psychol. 31, 264–268 (2012).
- M. E. Bauer, Chronic stress and immunosenescence: A review. Neuroimmunomodulation 15, 241–250 (2008).
- A. Sommershof et al., Substantial reduction of naïve and regulatory T cells following traumatic stress. Brain Behav. Immun. 23, 1117–1124 (2009).
- M. Maes et al., The effects of psychological stress on leukocyte subset distribution in humans: Evidence of immune activation. Neuropsychobiology 39, 1–9 (1999).
- M. M. C. Elwenspoek et al., T cell immunosenescence after early life adversity: Association with cytomegalovirus infection. Front. Immunol. 8, 1263 (2017).
- J. A. Bosch, J. E. Fischer, J. C. Fischer, Psychologically adverse work conditions are associated with CD8+ T cell differentiation indicative of immunesenescence. Brain Behav. Immun. 23, 527–534 (2009).
- A. E. Aiello et al., Income and markers of immunological cellular aging. Psychosom. Med. 78, 657–666 (2016).
- A. E. Aiello et al., PTSD is associated with an increase in aged T cell phenotypes in adults living in Detroit. Psychoneuroendocrinology 67, 133–141 (2016).
- K. E. Rentscher et al., Chronic stress exposure and daily stress appraisals relate to biological aging marker p16INK4a. Psychoneuroendocrinology 102, 139–148 (2019).
- C. P. Fagundes, R. Glaser, J. K. Kiecolt-Glaser, Stressful early life experiences and immune dysregulation across the lifespan. Brain Behav. Immun. 27, 8–12 (2013).
- J. K. Kiecolt-Glaser, L. McGuire, T. F. Robles, R. Glaser, Psychoneuroimmunology: Psychological influences on immune function and health. J. Consult. Clin. Psychol. 70, 537–547 (2002).
- J. K. Kiecolt-Glaser, R. Glaser, Stress and immunity: Age enhances the risks. Curr. Dir. Psychol. Sci. 10, 18–21 (2001).
- H. T. Maecker, J. P. McCoy, R. Nussenblatt, Standardizing immunophenotyping for the Human Immunology Project. Nat. Rev. Immunol. 12, 191–200 (2012).
- R. J. Turner, B. Wheaton, D. A. Lloyd, The epidemiology of social stress. Am. Sociol. Rev. 60, 104–125 (1995).
- W. M. Troxel, K. A. Matthews, J. T. Bromberger, K. Sutton-Tyrrell, Chronic stress burden, discrimination, and subclinical carotid artery disease in African American and Caucasian women. Health Psychol. 22, 300–309 (2003).
- D. R. Williams, Yan Yu, J. S. Jackson, N. B. Anderson, Racial differences in physical and mental health: Socio-economic status, stress and discrimination. J. Health Psychol. 2, 335–351 (1997).
- N. Krause, B. A. Shaw, J. Cairney, A descriptive epidemiology of lifetime trauma and the physical health status of older adults. Psychol. Aging 19, 637–648 (2004).
- E. M. Crimmins, J. D. Faul, B. Thyagarajan, D. R. Weir, Venous Blood Collection and Assay Protocol in the 2016 Health and Retirement Study 2016 Venous Blood Study (VBS, 2017).
- A. Aiello et al., Immunosenescence and its hallmarks: How to oppose aging strategically? A review of potential options for therapeutic intervention. Front. Immunol. 10, 2247 (2019).